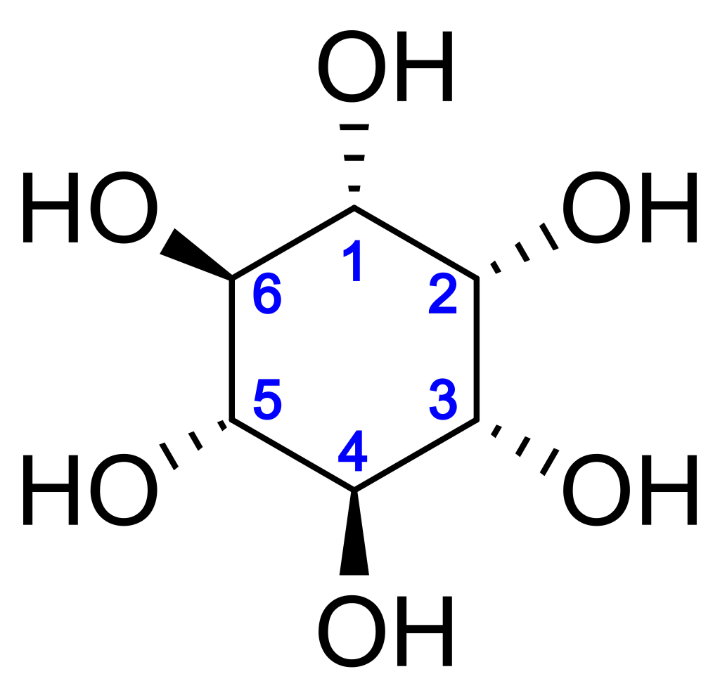Introduction: Rethinking How Complex Molecules Are Made
The global biotech sector is undergoing a transformation—one driven by sustainability, precision, and the need for alternatives to energy-intensive chemical processes. At the center of this shift is a renewed focus on metabolically significant molecules that can influence health, signal transduction, and cellular balance across a wide range of organisms. Among these, myo-inositol has emerged as a molecule of both scientific fascination and industrial relevance.
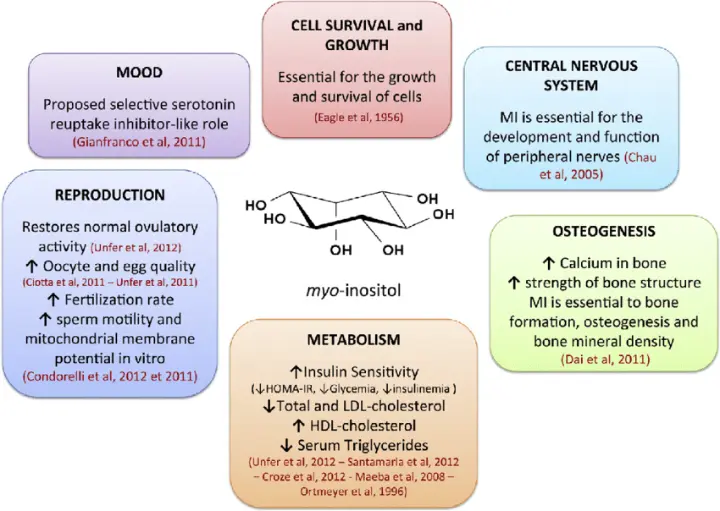
Once primarily recognized for its structural role in cellular signaling, myo-inositol is now being reevaluated as a strategic biotechnological target—especially in therapeutic, nutritional, and diagnostic applications. From regulating insulin sensitivity and lipid metabolism to improving mental health outcomes and reproductive function, the bioactivity of myo-inositol has elevated its importance in both research and applied innovation.
But how can such a molecule once difficult to extract or synthesize at scale—be efficiently produced in a way that meets today’s environmental and industrial standards? Recent advances in enzyme-driven biotechnology provide a compelling answer.
🔬 Synthetic Biology and Enzyme Systems
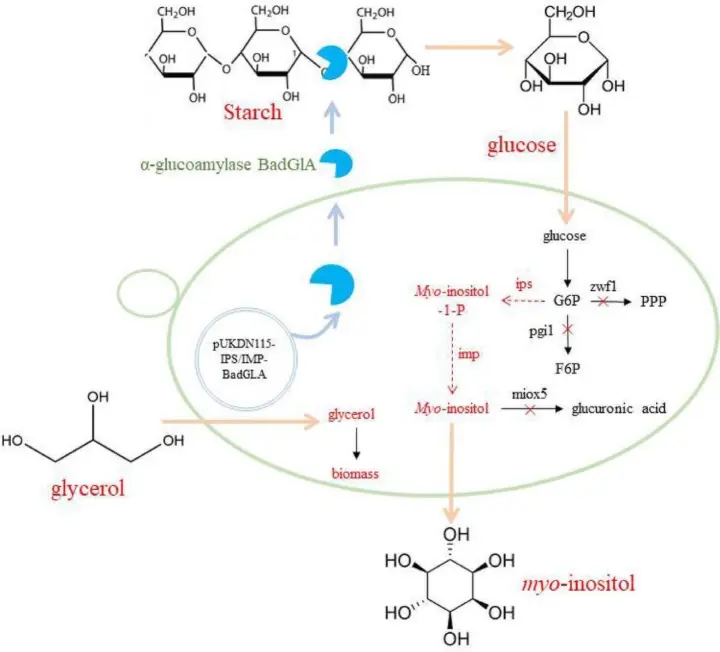
A breakthrough has recently been reported in Nature Catalysis, where researchers developed a fully cell-free biosynthetic platform capable of producing myo-inositol at an industrial scale of over 20,000 liters. Unlike traditional systems, this method leverages a synthetic enzymatic cascade that mimics key steps in natural metabolism but does so entirely outside of living cells.
This innovative method not only avoids genetic modification and fermentation but also achieves near-complete conversion efficiency and minimal byproduct formation. At its core lies the power of hyperthermophilic enzymes—proteins sourced from thermophilic organisms that retain catalytic activity at elevated temperatures, increasing both reaction rate and stability.
🧠 How Myo-Inositol is Made from Starch
The new production system starts with starch-derived glucose, a renewable and abundant carbohydrate. This is broken down through a series of enzymatic steps designed to:
- Hydrolyze starch into glucose monomers
- Phosphorylate and isomerize glucose into inositol precursors
- Cyclize and dephosphorylate intermediates to produce high-purity myo-inositol
Each enzyme in the pathway is carefully selected and engineered for specificity, efficiency, and resilience. The entire cascade is optimized under controlled reaction conditions that maximize throughput and product quality, while minimizing waste.
This cell-free enzymatic system avoids the complexity of maintaining live microbial cultures and eliminates issues like plasmid loss, metabolic competition, and byproduct accumulation. It is a precision system—tailored to produce one molecule with high fidelity.
🌐 Applications Across Sectors
The scalability and purity of myo-inositol produced through this enzymatic process open the door to numerous applications across:
Medical and Pharmaceutical Fields
- Management of polycystic ovary syndrome (PCOS) and insulin resistance
- Adjunctive treatments in bipolar disorder and depression
-
Key component in lipid membrane research and cell signaling models
Nutraceuticals and Functional Foods
- Marketed as a metabolic health supplement
- Used in prebiotic formulations and sports recovery beverages
- Incorporated into functional food ingredients for targeted nutrition
Biotechnology and Research
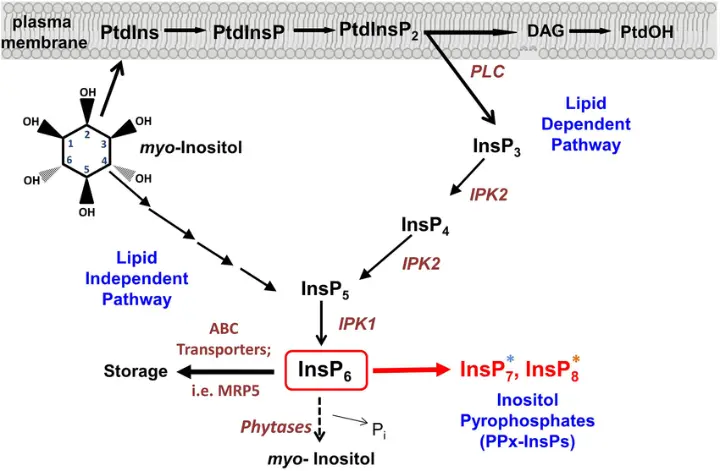
- Foundation for producing inositol phosphates, critical in cell signaling
- Substrate in biocatalysis platforms and metabolic flux studies
- Scaffold for the design of biosensors, diagnostics, and nanodevices
The versatility of this molecule is matched only by the possibilities that enzyme-based production offers. In short: myo-inositol is now accessible, affordable, and adaptable.
✨ Future Outlook
As the biotechnology industry continues to shift toward enzyme-driven processes, we may see similar methods applied to the production of rare sugars, sugar alcohols, and phosphorylated compounds. These techniques not only reduce environmental impact but also offer unprecedented control over molecular synthesis, enabling next-generation biopharmaceuticals, cell signaling research, and metabolic interventions.
With this milestone in myo-inositol manufacturing, we are witnessing how molecular biology, catalysis, and industrial design can converge to create solutions that are clean, scalable, and transformative.
For further information
Here you can find a current, comprehensive article on this topic:
An in vitro synthetic biology platform for the industrial biomanufacturing of myo-inositol from starch